Introduction: Clinical guidelines and practice have shifted toward increasing use of novel Bruton's tyrosine kinase inhibitors (BTKi) or B-cell lymphoma 2 inhibitor (BCL2i) for treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). To highlight recent changes in clinical practice, this study evaluated real-world treatment sequences, with a focus on sequential novel targeted therapy, among patients with CLL/SLL in the US over time.
Methods: Adult patients with ≥2 medical claims for CLL/SLL diagnosis on distinct days and ≥1 claim for CLL/SLL therapy were identified in the Optum Clinformatics DataMart database (2014-2021). The index date was the date of initiation of the first observed line of therapy (LOT) following the first observed CLL/SLL diagnosis. The observation period spanned from index to the earliest of end of eligibility, data availability, or death. LOTs were identified using a claims-based algorithm adapted from published literature during the observation period. Each LOT spanned from the initiation of a new agent to discontinuation of all agents in the LOT (i.e., 90-day gap without any dispensing), a switch to another agent, or the addition of a new agent. The proportion of patients receiving sequential targeted therapy in the first 2 LOTs (i.e., BTKi/BCL2i as first-line [1L] and second-line [2L] treatments in any order) was described. For sequential BTKi in 1L and 2L, treatments were also described by agent (i.e., ibrutinib, acalabrutinib, zanubrutinib, and combinations thereof). To assess longitudinal patterns, Sankey plots were created for 2 temporal data cohorts: patients with a 1L in 2014-2017 and patients with a 1L in 2018-2021.
Results: We identified 7,146 eligible patients, 40.6% female, median age 73 years, with a median follow-up of 2 years after 1L initiation. The most common 1L treatment was BTKi (36.8%), then chemoimmunotherapy (CIT; 27.4%), CD20 (22.2%), chemotherapy (7.6%), and BCL2i (5.4%). In the first 2 LOTs, 22.3% of patients had sequential targeted therapy, comprising 70.0% BTKi→BTKi (i.e., BTKi retreated), 23.8% BTKi→BCL2i, 3.6% BCL2i→BCL2i, and 2.6% BCL2i→BTKi. The most common BTKi sequences by agent were ibrutinib→ibrutinib (61.6%) and ibrutinib→acalabrutinib (28.4%).
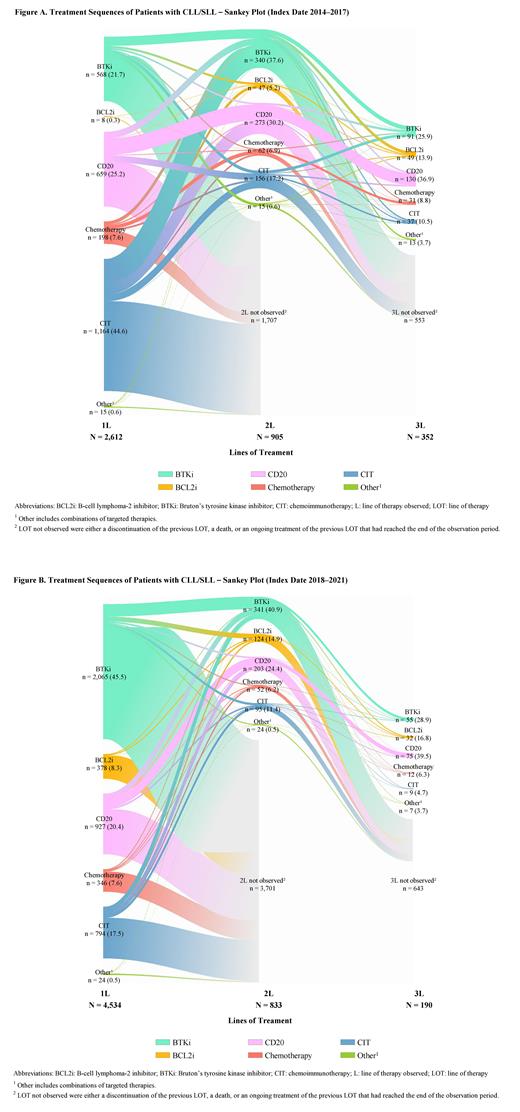
Among patients with a 1L in 2014-2017 (N=2,612; median follow-up=3 years; Figure A), the most common 1L treatment class was CIT (44.6%), followed by CD20 (25.2%), BTKi (21.7%), chemotherapy (7.6%), and BCL2i (0.3%). For those receiving a 2L treatment (N=905), the 3 most common treatment classes were BTKi (37.6%), CD20 (30.2%), and CIT (17.2%). The proportion of patients receiving sequential targeted therapy in the first 2 LOTs was 11.2%; among these patients, 80.2% had BTKi→BTKi; 17.8% had BTKi→BCL2i; 2.0% had BCL2i→BCL2i; and 0% had BCL2i→BTKi. The most common BTKi sequences by agent were ibrutinib→ibrutinib (81.5%) and ibrutinib→acalabrutinib (17.3%).
Among patients with a 1L in 2018-2021 (N=4,534; median follow-up=1 year; Figure B), the most common 1L treatment class was BTKi (45.5%), followed by CD20 (20.4%), CIT (17.5%), BCL2i (8.3%), and chemotherapy (7.6%). For 2L (N=833), the 3 most common treatment classes were BTKi (40.9%), CD20 (24.4%), and BCL2i (14.9%). The proportion of patients receiving sequential targeted therapy in the first 2 LOTs was 34.3%, comprising 66.4% BTKi→BTKi; 25.9% BTKi→BCL2i; 4.2% BCL2i→BCL2i; and 3.5% BCL2i→BTKi). Among those receiving sequential BTKi, the most common sequences by agent were ibrutinib→ibrutinib (53.2%), ibrutinib→acalabrutinib (33.2%), and acalabrutinib→acalabrutinib (5.3%).
Conclusions: This longitudinal real-world study found a substantial increase in patients with CLL/SLL receiving targeted therapies in 1L over time. In particular, the proportion receiving BTKi in 1L doubled in 2018-2021 relative to 2014-2017 and accounted for almost half of the recent 1L CLL/SLL initiations. A third of patients with 1L observed in 2018-2021 also received a targeted agent in 2L, relative to about 1 in 10 patients in 2014-2017, with BTKi→BTKi being the most common sequence. Surprisingly, patients who received a BTKi in 1L were more likely to receive a BTKi in 2L than they were to switch to a BCL2i. Future studies should assess clinical outcomes to determine optimal sequences for CLL/SLL as well as the reasons for re-treatment with BTKi.
Disclosures
De Nigris:Merck & Co., Inc.: Current equity holder in publicly-traded company; Merck Sharp & Dohme LLC (a subsidiary of Merck & Co): Current Employment. Cheng:Analysis Group, Inc.: Current Employment. Sarpong:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Leng:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Farooqui:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Agu:Abbvie: Other: Internship; Merck & Co., Inc.: Other: Internship. Catillon:Analysis Group, Inc.: Current Employment. Lejeune:Groupe d'analyse, Ltée: Current Employment. Downes:Analysis Group, Inc.: Current Employment. Matay:Merck & Co., Inc.: Research Funding. Duh:Analysis Group, Inc.: Current Employment. Huntington:AbbVie: Consultancy; Bayer Healthcare: Consultancy; BeiGene USA, Inc.: Consultancy; Epizyme, Inc.: Consultancy; Genentech: Consultancy; Janssen Pharmaceuticals: Consultancy; Lilly USA, LLC: Consultancy; Merck: Consultancy; Novartis: Consultancy; Pharmacyclics LLC, An AbbVie Company: Consultancy; Seagen Inc.: Consultancy; Servier Pharmaceuticals LLC: Consultancy; TG Therapeutics: Consultancy; Tyme Inc: Consultancy; AstraZeneca: Consultancy; Arvinas: Consultancy; ADC Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal